Ozempic, a prescription drug designed to treat diabetes, has been in short supply for much of 2023 after becoming popular for its potential weight loss benefits.
VERIFY reader Shanta texted us to ask if there is fake or counterfeit Ozempic being sold in the United States. Others have asked in online forums if they’ve recently bought fake Ozempic, such as this Dec. 15 post to Reddit.
THE QUESTION
Is there counterfeit Ozempic in the U.S.?
THE SOURCES
Novo Nordisk, the manufacturer of Ozempic
GoodRx, a website that helps people get discounts on medication
THE ANSWER
Yes, counterfeit Ozempic has been sold in the United States.
WHAT WE FOUND
Novo Nordisk, the manufacturer of Ozempic, and the Food and Drug Administration (FDA) have been issuing warnings about counterfeit Ozempic in the U.S. since June 2023.
On June 16, 2023, Novo Nordisk issued a press release alerting the public that a counterfeit Ozempic pen was found in the United States. The counterfeit pen was reportedly purchased from a retail pharmacy and contained another kind of diabetes medication different from Ozempic.
Ozempic is a medication containing semaglutide, which mimics a hormone that prompts the body to produce more insulin and reduces appetite in the brain. Novo Nordisk said the counterfeit version of Ozempic contained insulin glargine, which is used in the diabetes medication Lantus.
Ozempic has been in short supply since 2022 after it gained popularity for its potential weight loss benefits, though it has not been approved by the FDA as a weight loss drug. GoodRx, a website that helps people get discounts on medication, warns consumers about counterfeit Ozempic that has hit the market “given recent increased demand and short supply.”
Since the June 16 warning issued by Novo Nordisk, the National Association of Boards of Pharmacy (NABP) has issued two warnings regarding counterfeit Ozempic. Most recently, the FDA announced on Dec. 21 that it has seized thousands of units of counterfeit Ozempic found in the “legitimate U.S. drug supply chain.” The seized counterfeits are labeled with lot number NAR0074 and serial number 430834149057, the FDA said.
The FDA said some counterfeit Ozempic products may still be available for purchase, but didn’t specify where.
The warnings have included both products falsely passed off as Ozempic, and unapproved medication containing semaglutide such as knock-offs sold online in generic vials. Some of the unapproved medication includes salt forms of semaglutide, which is not the same active ingredient as the base form of semaglutide used in Ozempic.
The “legitimate U.S. drug supply chain” is the pipeline of medications produced by drug manufacturers, then distributed to retail pharmacies and finally sold to consumers. This supply chain is regulated by the FDA, giving the agency the ability to detect and remove from the supply any drug that is counterfeit, stolen, contaminated or otherwise harmful.
Since the FDA can monitor this legitimate drug supply chain and potentially detect counterfeits, you’re less likely to end up with counterfeit Ozempic if you get it through a legitimate, state-licensed pharmacy than you are if you get it from online marketplaces, unlicensed retailers and foreign pharmacies.
The FDA recommends consumers obtain Ozempic only with a valid prescription and through state-licensed pharmacies for this reason.
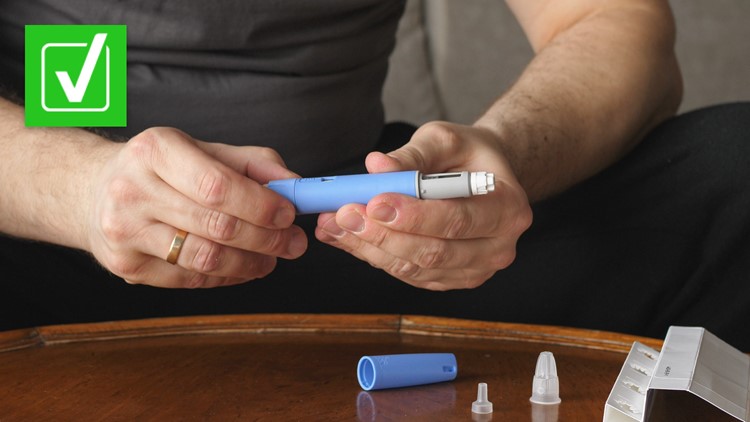
How to spot counterfeit Ozempic
There are some visible clues you can use to tell the difference between a real Ozempic pen and a counterfeit.
GoodRx has published detailed descriptions and photos of what real Ozempic pens with red, blue and yellow labels look like. Novo Nordisk says genuine Ozempic pens do not extend or increase in length when setting the dose, while a counterfeit pen may. The labels on counterfeit Ozempic pens could be of poor quality and may not adhere well to the pen. A counterfeit carton may have spelling mistakes on the box, and may not include a tampering resistant or perforation.
The FDA published photos of authentic Ozempic needles and counterfeit needles:




“The safety or efficacy of counterfeit products cannot be assured and they should not be used,” Novo Nordisk said. “Potential risks of taking a counterfeit medicine include serious adverse events.”
The FDA reported five adverse events from counterfeit Ozempic products in its December press release, although none of the events were serious. The FDA said the adverse events were consistent with known common adverse reactions to authentic Ozempic, including nausea, vomiting, diarrhea, abdominal pain and constipation. In November, CBS News reported that there have been at least three hospitalizations in the U.S. related to Ozempic counterfeits.
Counterfeit Ozempic has not been exclusively a U.S. problem. On Oct. 18, 2023, the European Medicines Agency issued a press release warning of counterfeit Ozempic pens distributed in Germany. The Partnership for Safe Medicines reports counterfeit Ozempic has been found in at least 16 countries spread across North America, Europe, Asia, Africa and Oceania.